Acetylén
Acetylene (ethine) is a hydrocarbon belonging to the group of alkynes. Its chemical formula is C2H2, which means it consists of two carbon atoms and two hydrogen atoms. It is a colored and colorless gas with a specific smell. Acetylene is known for its triple bond between carbon atoms, which makes it relatively reactive and suitable for various chemical processes.
Definition and Uses of Acetylene:
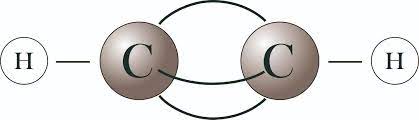
Chemical formula: C2H2
Structure: Acetylene has a linear molecular structure with two carbon atoms joined by a triple bond.
Use of acetylene:
Oxygen-acetylene welding and cutting: You might have heard of the so-called "acetylene flame," which is used in the process of oxygen-acetylene welding and metal cutting. Acetylene is burned in air in the presence of oxygen, creating a very hot flame with temperatures reaching thousands of degrees Celsius. This flame is suitable for welding metals and cutting materials.
Chemical production: Acetylene is used as a raw material in the production of various chemicals, such as ethylene (from which plastic products are made), acetaldehyde (a chemical used in the chemical industry), and other organic compounds.
Production of polymers: It is an important source for the production of polymers, which are the basis for the production of various plastic materials.
Lighting: In the past, acetylene was used for lighting in gas lamps and lamps, as its combustion produces intense light.
Production of plastics: Acetylene is used in the production of various plastic materials that are widely used in various industries.
Laboratory research: Acetylene is also used in laboratory conditions for the synthesis of organic molecules and the study of chemical reactions.
It is important to note that acetylene is a relatively sensitive and reactive gas in terms of handling and storage, so it is necessary to work with it with proper expertise and safety.
