Electroplating
Electroplating is the process of electrochemically plating a metallic material, often using a metal such as zinc, nickel or chromium. This process is carried out in order to protect the surface of the metal from corrosion, improve the aesthetic appearance, increase resistance and other properties of the surface.
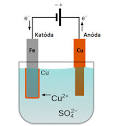
The electroplating process usually uses an electrolyte (a soluble chemical solution containing metal ions) where the object to be plated (referred to as the anode) is connected to the negative pole of an electrical circuit and the metal material to be deposited on the surface of the object (referred to as the cathode ), is connected to the positive pole. The electric current causes metal ions to migrate from the electrolyte to the object's surface, where they deposit and form a thin layer of new metal.
Electroplating can be done with different metals depending on the desired surface properties and application. The most common types of galvanization include:
Zinc Plating: Zinc is applied to the surface of the metal to protect it from corrosion. It is used, for example, in the production of tanks, pipes and metal structures.
Nickel plating: A nickel layer is applied, which can improve the corrosion resistance, hardness and aesthetic appearance of the surface. It is used in the manufacture of automobiles, electronic equipment and jewelry.
Chrome plating: Chrome is applied to the surface, which gives a high shine and increased durability. It is used in the production of furniture, appliances and automobiles.
Galvanization is a widely used process in industry that allows the creation of protective and decorative coatings on metal surfaces.
