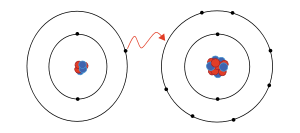
ion
An ion is an atom or molecule that has an electrical charge because it has a different number of electrons and protons. Atoms are the basic building blocks of matter, and when they have the same number of electrons as protons, they are electrically neutral. But when an atom gains or loses one or more electrons, it becomes ionized and gains an electrical charge.
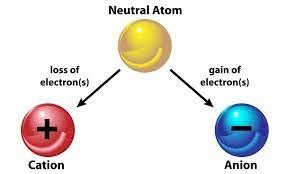
There are two types of ions:
Cations: These are ions that have a positive electrical charge because they have lost one or more electrons compared to their neutral state. Cations are formed from typically metallic elements.
Anions: These are ions that have a negative electrical charge because they have gained one or more electrons compared to the neutral state. Anions are often formed from non-metallic elements.
Ions are fundamental to chemistry and physics because their electrical charge affects their behavior in chemical reactions, electric fields, and electromagnetic interactions.